News and articles
News of major activities and announcements from across the department, plus articles featuring the people, projects and programs driving economic growth and prosperity across Victoria.
Showing 1 to 12 of 162 items.

Winners celebrated at the 2023-24 Premier’s Awards for Health and Medical Research
Up and coming leaders in health and medical research were recognised at the 2023-24 Premier’s Awards for Health and Medical Research on Tuesday 23 April 2024.

New non-stop flights to South Korea take off from Victoria
New seasonal flights between Melbourne Airport and Seoul Incheon International Airport will operate four times per week between July and September 2024.

The sky’s the limit for the future of sustainable air travel
Dovetail Electric Aviation’s new Development Centre has opened in the Latrobe Aerospace Technology Precinct, located at Latrobe Regional Airport.
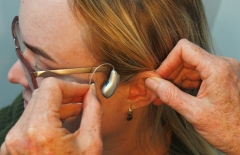
Victorian research finds hearing aids boost brain function
Hearing aid users experience benefits to their brain function according to new results from Victorian medical researchers.

Get creative these holidays
School holidays can be an expensive time, but when you live in the creative state, you don’t have to spend big to keep the kids entertained.

Visit regional Victoria this Easter
The Easter holidays are a great time to get out and explore our wonderful state. Head to Bells Beach for the iconic Rip Curl Pro, cheer on the runners at the Stawell Gift or step back in time for a ride on a heritage steam train.

Elite wheelchair athletes take Melbourne by storm
The Wheelchair Rugby National League Melbourne Invitational returned to the city’s west for a battle of the best.

Victoria’s biggest VET student survey is underway
Almost 180,000 students are being asked to provide feedback on their course through the Student Satisfaction Survey, the biggest survey of its kind in Victoria.

Health Select Services transports regional communities to better health
Jeanette Cunningham from Health Select Services strives to make a difference to the health of regional and rural Victorians in a big way.

Make a difference to Victoria’s training and skills system
The Victorian Government invites applications for part-time positions on TAFE Boards.

The ability to embrace disability
Rebecca Hope, Victorian Trainee of the Year 2023, shares how having a disability is a strength.

Australian art and fashion icons unite for new exhibition
Lisa Gorman + Mirka Mora: To breathe with the rhythm of the heart, at Warrnambool Art Gallery, brings two Australian fashion and art icons together.
